The basic difference between the two is – osmosis is the speed of solvent (water) from a region of high concentration through a semiconductor membrane to maintain equilibrium in a region of low concentration. On the other hand, diffusion can be described as the circulation of molecules (solid, liquid or gases) from a region of high concentration to a region of low concentration, but not through a semiconductor membrane.
Both of such examples are examples of passive transport. It is a natural process occurring inside the body and thus it promotes the movement of molecules without the need for energy. The motion can occur either through low concentration or from high concentration to low, and this difference in concentration of particles is called concentration gradient.
This process is performed to equalize the concentration gradient on either side of the membrane, especially in the case of water (solvent). In the following material, we will consider the important differences between the two types of motion, followed by a brief discussion.
What is an osmosis?
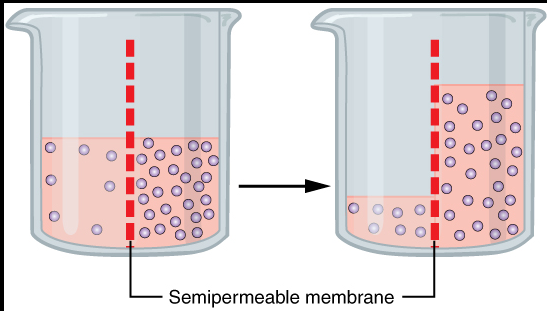
The function of osmosis is to maintain equilibrium on both sides of the membrane and therefore in this process the only motion of the water molecule is also known as solvent.
To maintain homeostasis, water molecules move from the edge to the area of higher water density with lower water concentrations. And also from low solute concentrations to high solute concentrations. In particular, water molecules are passed through a semi-permeable membrane. So we can say that osmosis is a special type of propagation.
Osmosis is important in the distribution of nutrients and in a release of metabolic waste from the body, and in maintaining the concentration gradient inside and outside the cell.
Osmosis in plants is helpful in absorbing water from the soil, it helps maintain water levels, even at the time of water loss, from cell to cell proliferation, providing mechanical support.
Factors affecting osmosis are:
Diffusion distance
Concentration gradient.
Temperature.
Osmotic pressure
What is diffusion?

Molecules such as solids, gases, or liquid move from a region of high concentration to a region of low concentration. The reason for this movement is the randomly moving molecules present in high concentrations, which have free energy, and when they move to the region of low concentration, the gain of free energy as well as equilibrium of the diffusing molecule is achieved. Semi-permeable membranes have no role.
Thus, diffusion is important in making energy. It helps in the exchange of gases in animals during respiration. It is also helpful in the process of evaporation and photosynthesis in plants.
Example: If a drop of blue ink is poured into a jar filled with water, the ink will be distributed evenly throughout the water, and the particles will be distributed everywhere, this being the simplest example of diffusion.
Another example to explain diffusion is any spray of deodorant, such as perfume, so when they are used or opened, the gas molecules get distributed evenly over a certain available space.
| Osmosis | Diffusion |
| Asymmetrical seminal matter (solvent) is used to transport water from a region of high concentration to a region of low concentration, called osmosis through a semiconductor membrane. | The motion of (solid, liquid or gases), but not necessarily through a semi-permeable membrane, is called diffusion. |
| Semi-permeable membranes move through semi-permeable membranes. | The motion is direct and semi-permeable membranes are not required. |
| This process takes place in a liquid medium. | This process occurs in any medium (solid, liquid and gases). |
| Movement is basically of the solvent (water). | Movement can be in solid, liquid or gases. |
| The rate of process osmosis is a slow process. | Diffusion is a fast process. |
| Free energy osmosis is dependent on the reduction of free energy from one solvent to another. | It is the speed of molecules from their higher free energy fields to lower free energy fields. |
Conclusion
So in general terms, we can say that the interaction of molecules to maintain equilibrium is a naturally occurring process in the body, and is called osmosis and diffusion. However this physiological process is sometimes misleading. But there is an important value in science.





