Here, in this blog post, we are going to explore what is a compound, different types of compounds, and some of the examples of compounds.
A compound is a pure element that is formed of the chemical combinations of two or more elements. A compound is usually formed when two or more chemical elements are chemically bonded together. In mixtures, the substances are not chemically bonded together.
In compounds, two or more elements are chemically bonded in a fixed ratio by mass, the new product is formed. When two or more elements are combined, some individual property of the elements is lost and the new compound is formed with new properties.
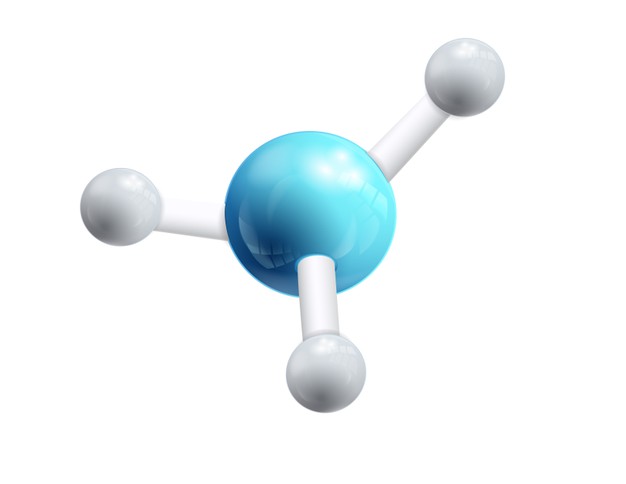
Water molecule
Water: In water, two hydrogen atoms are combined to an oxygen atom chemically.
The different kinds of bonds holding elements together in a compound can vary: two common types of bonds are called covalent bonds and ionic bonds.
The elements in any compound are always bonded in fixed ratios.
Chemical Formula:
Compounds are denoted by their chemical formula. The symbolic representation of the ratios of atoms that forms a particular chemical compound is known as Chemical Formula.
Examples of Compounds
Example 1:
Pure water is a compound formed from two elements: hydrogen and oxygen. The ratio of hydrogen to oxygen in water should be 2:1. Each molecule of water comprises two hydrogen atoms bonded to a single oxygen atom.
Example 2:

Pure table salt is a compound formed from two elements: sodium and chlorine. The ratio of sodium ions to chloride ions in sodium chloride must be 1:1.
Example 3:

Pure methane is a compound formed from two elements: carbon and hydrogen. The ratio of hydrogen to carbon in methane should be 4:1.
Example 4:
Pure glucose is a compound formed from three elements: carbon, hydrogen, and oxygen. The ratio of hydrogen to carbon and oxygen in glucose should be 2:1:1.
Usually, compounds are decomposed chemically into their constituent elements.
Some Compounds Examples with their Molecular Formula:
Water – Formula: H2O = Hydrogen2 + Oxygen.
Hydrogen Peroxide – Formula: H2O2 = Hydrogen2 + Oxygen2
Salt – Formula: NaCl = Sodium + Chlorine
Baking Soda – Formula: NaHCO3 = Sodium + Hydrogen + Carbon + Oxygen3
Octane – Formula: C8H18 = Carbons 8 + Hydrogen18
Alcohol – Formula: C2H6O = Carbons 2 + Hydrogens 6 + Oxygen
Acetic Acid – Formula: C2H4O2 = Carbons 2 + Hydrogens 4 + Oxygen 2
Sulphuric Acid – Formula: H2SO4 = Hydrogens 2 + Sulphur + Oxygen 4
Ammonia – Formula: NH3 = Nitrogen + Hydrogens 3
Methane – Formula: CH4 = Carbon + Hydrogens 4
Types of Compounds
Metal + Nonmetal —> ionic compound (usually)
Metal + Polyatomic ion —> ionic compound (usually)
Nonmetal + Nonmetal —> covalent compound (usually)
Hydrogen + Nonmetal —> covalent compound (usually)
Compounds can be categorized into two types. They are molecular compounds and salts. In molecular compounds, two or more atoms are bonded with each other through covalent bonds. In salts, two or more atoms are held together with ionic bonds. These are the two types of bonds out of which every compound is usually formed.
In Ionic bonds, two or more atoms transfer electrons to each other but in covalent bonds, atoms consisting of the same electronegativity share their electrons.
In covalent bonds, atoms neither attract each other nor repel the shared electrons.
Ionic bonds are formed when a nonmetallic substance and a metallic substance share their electrons. While covalent bonds are formed when two nonmetallic substances share their electrons.
Conclusion
We discussed what is a compound and some examples of compounds with their molecular formula. If you want to ask any questions regarding this topic, then please let us know in the comments section. We will be glad to clear the concepts.





One Comment
Nice information sir good very interesting knowledge via your websit thank you